You are currently viewing documentation for CompuTec ProcessForce 3.0, which is a plugin installed and managed through CompuTec AppEngine 3.0, built on .NET 8.
If you are working with older environments, you may need the documentation for CompuTec ProcessForce 2.0, which is still supported: https://learn.computec.one/docs/processforce/2.0/.
Please note that CompuTec AppEngine 3.0 introduces a new architecture. All CompuTec components, including the CompuTec ProcessForce 3.0 plugin, must be updated together to ensure full compatibility.
Before starting your installation or upgrade process, we strongly recommend reviewing the CompuTec AppEngine 3.0 and CompuTec ProcessForce 3.0 documentation.
Quality Control Configuration
The Quality Control Configuration section allows users to define the essential settings for conducting quality tests. This includes configuring Document Series Numbers, defining resources, and managing item information for the testing process. Proper configuration ensures seamless and accurate quality control processes across various testing activities.
This section provides a structured guide to setting up quality control settings in CompuTec ProcessForce. Use this page to understand, configure, and manage resources and items used in quality testing within SAP Business One.
General Settings
Before setting up QC components, ensure that the Quality Control feature is activated and properly defined under system settings.
➡️ Learn more about Quality Control.
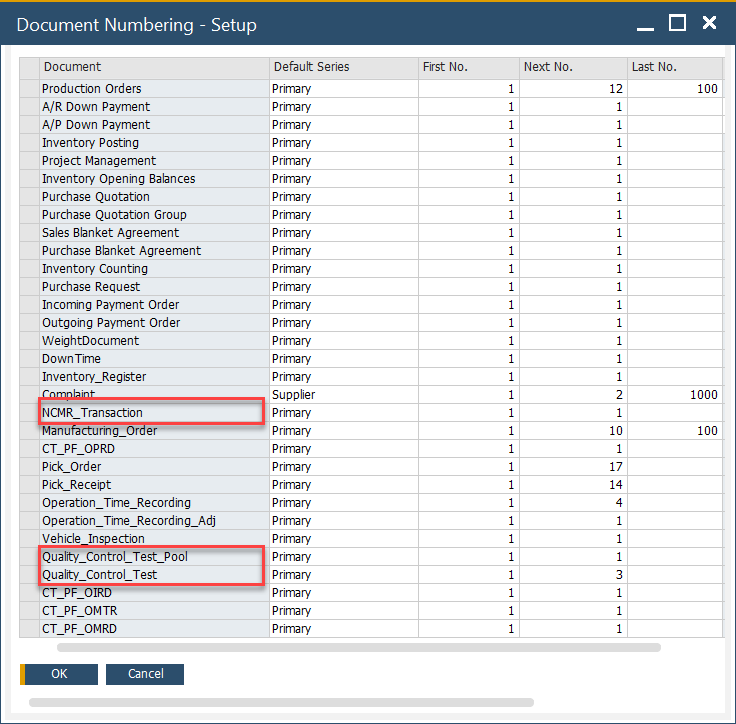
Document Series Number
The following Quality Control forms use SAP Business One Document Numbering:
Resources
Resources refer to testing tools and equipment like microscopes, gauges, or X-ray machines. These are configured to appear in QC-related forms using specific filters in Item Master Data.
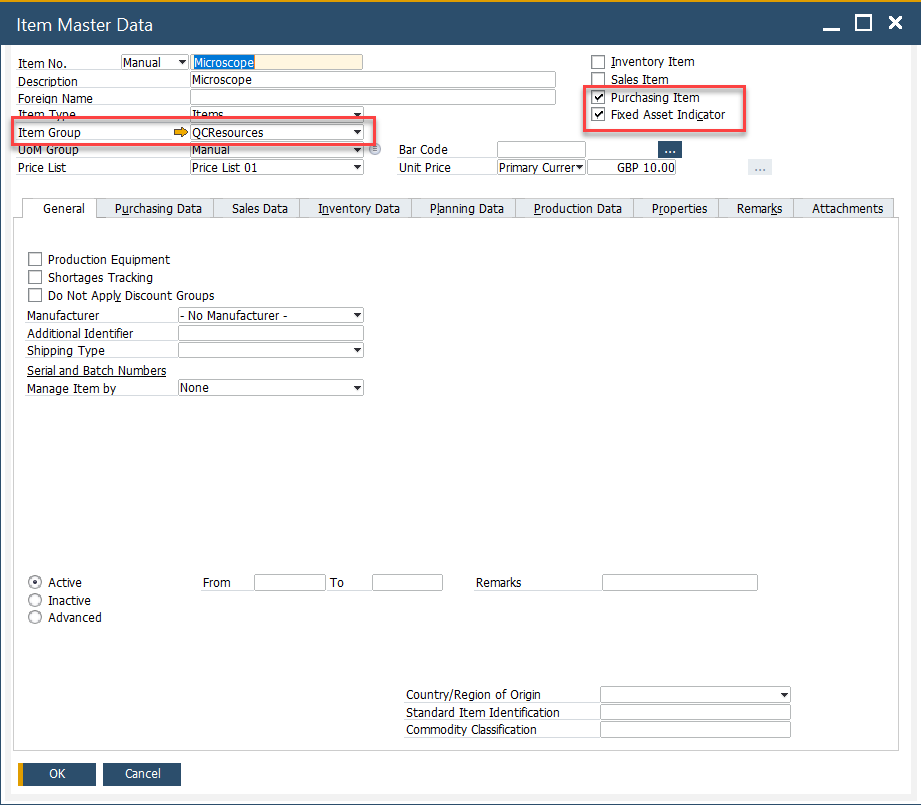
To define a resource:
-
Check both Purchase Item and Fixed Assets fields in the item master.
-
Assign the item to a designated Item Group (e.g., “QC Equipment”).
-
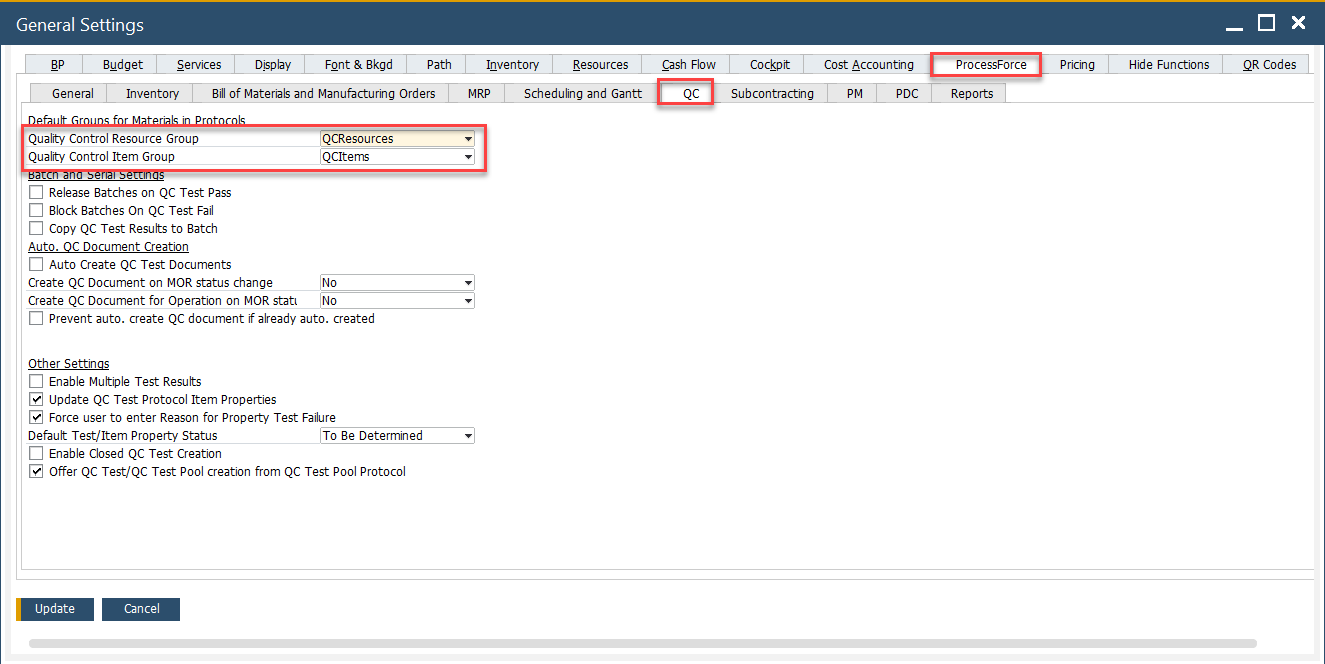
Go to General Settings > ProcessForce Tab.
-
Set that Item Group under QC Resource Group.
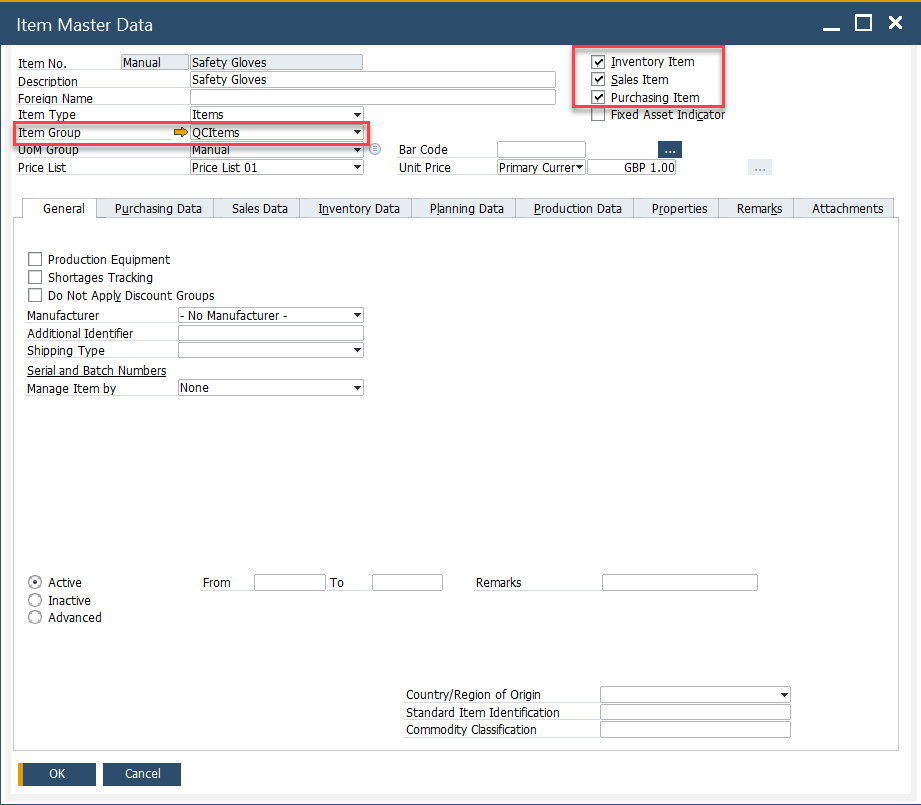
Items
The Test Protocol and Quality Control Test forms also allow users to define specific items related to quality control, such as safety glasses, gloves, or chemicals used only within the Quality Control process and department. These forms filter the Item Master Data based on the following criteria:
-
The Inventory, Sales, and Purchase Item fields are checked.
-
Assign the item to a specific Item Group (e.g., “QC Consumables”).
-
In General Settings > ProcessForce Tab, assign this group to QC Item Group.